|
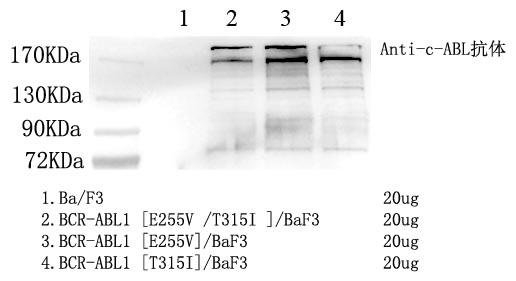
Presence of a BCR-ABL1 fusion gene is necessary for the pathogenesis of CML. In up to 95% of cases, a t(9;22) (q34;q11) translocation results in the BCR-ABL1 fusion gene (Faderl et al. 1999). This translocation results in the Philadephia chromosome. In rare CML cases lacking the traditional t(9;22) translocation, other translocations result in the creation of the BCR-ABL1 fusion gene, which sometimes involve multiple chromosomes.
ABL1 is a tyrosine kinase, and, in normal cells, it plays a role in cellular differentiation and regulation of the cell cycle. The BCR-ABL1 fusion gene creates a constitutively active tyrosine kinase, which leads to uncontrolled proliferation.
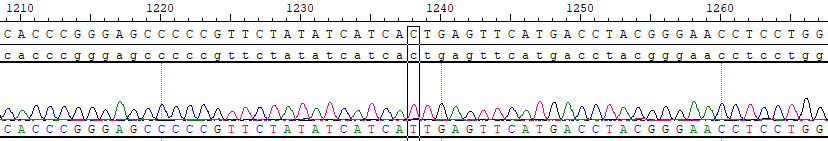
Imatinib is the first-generation ABL tyrosine kinase inhibitor, and it was approved by the FDA in 2001; label indications for CML include use in newly diagnosed adult and pediatric patients and in patients after failure of interferon-alpha therapy. While treatment responses to imatinib are often dramatic and lasting, 30–40% of patients will eventually need further treatment (Santos et al. 2011). In many but not all cases, this is due to the acquisition of point mutations in the tyrosine kinase domain of the BCR-ABL1 fusion gene, which renders the protein insensitive to the inhibitory effect of imatinib. This type of disease progression led to the development of second-line TKIs: dasatinib, nilotinib, and bosutinib. Dasatinib and bosutinib have the additional advantage of being inhibitors of SRC.
The second-generation TKIs, dasatinib, nilotinib, and bosutinib, are more potent than imatinib, and they were developed to treat cases of CML resistant to imatinib. Dasatinib and nilotinib are approved for use in CML in newly diagnosed adults, while dasatinib, nilotinib, and bosutinib are approved for use in adults with resistance or intolerance to prior therapy that included imatinib (FDA 2012). Soverini et al. (2011) made mutation-specific treatment decision recommendations that were adopted by NCCN (2012?). Recommendations based on preclinical data are as follows: for T315I, HSCT or clinical trial; for V299L, T315A, and F317L/V/I/C, consider nilotinib rather than dasatinib; for Y253H, E255K/V, and F359V/C/I, consider dasatinib rather than nilotinib; and for all other mutations, consider high-dose imatinib, dasatinib, or nilotinib.
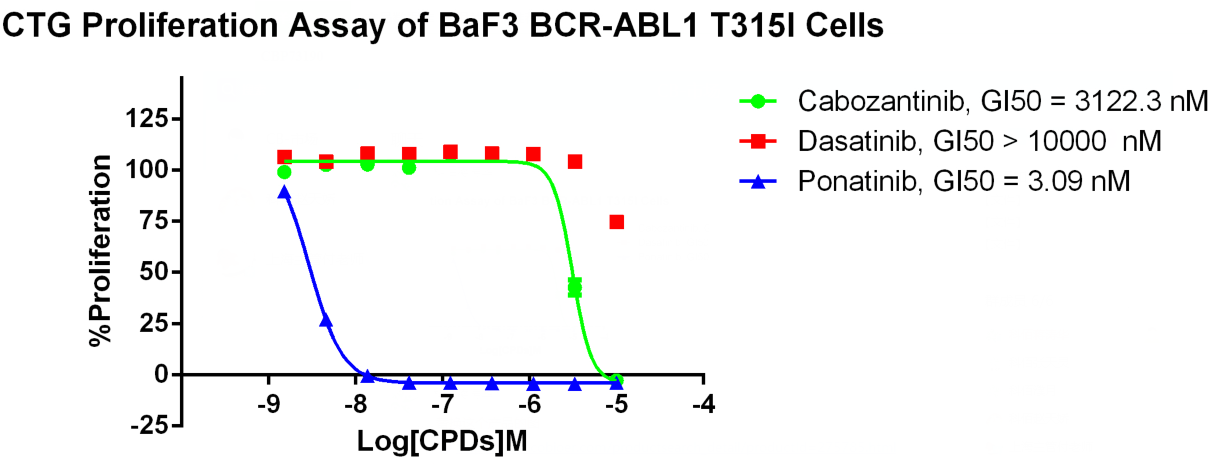
Ponatinib is a third-line TKI, developed specifically to address imatinib resistance due to the BCR-ABL1 T315I resistance mutation. Ponatinib is currently being investigated in phase III clinical trials.
|